
Imagine cleansing wastewater of a harmful yet valuable element while, at the same time, helping to satisfy the world’s growing demand for high-performance batteries.
Transformative research at the Joint School of Nanoscience and Nanoengineering, or JSNN, promises to do just that. The project is led by Associate Professor Hemali Rathnayake, assisted by graduate student Sheeba Dawood.
The element lithium is in great demand as a primary component in long-lasting batteries that power everything from smartphones to Tesla electric vehicles.
“There is a huge market for lithium for energy storage applications,” Rathnayake says. Demand is so high that the light-colored substance has been called “white gold.”
The world’s largest lithium deposits are in Australia, with other large concentrations in South America. Yet trace amounts of lithium are quite common, especially in wastewater that is a byproduct of extracting petroleum from the earth.
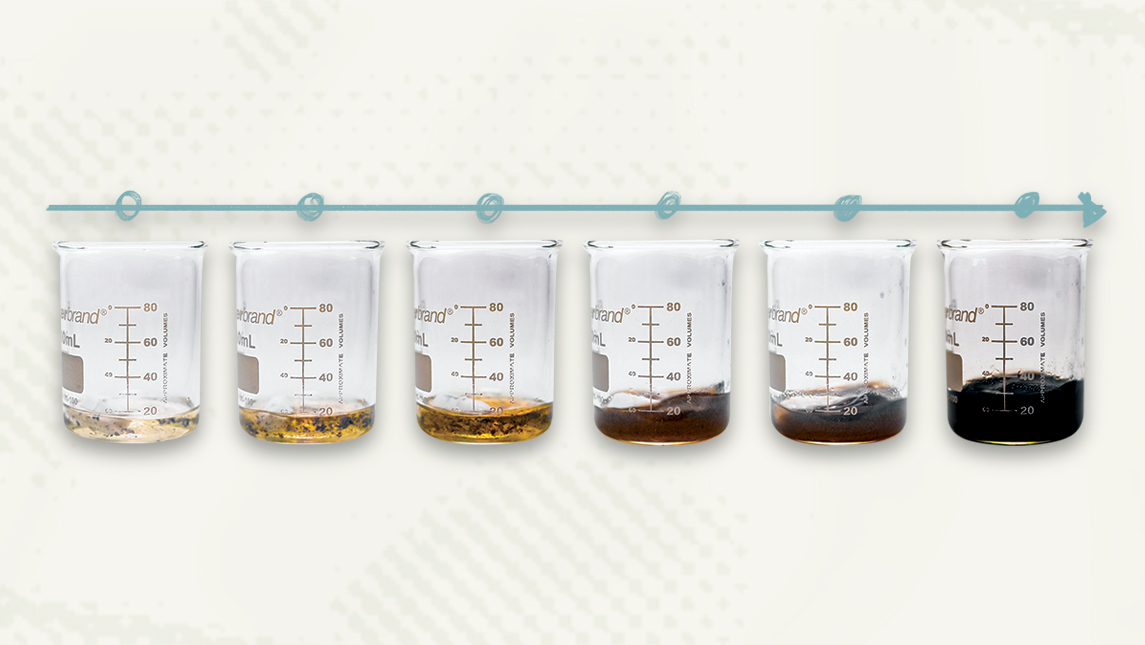
The traditional method of removing this lightweight metal from petroleum wastewater utilizes evaporating settling ponds, a process that can take up to two years. And that’s just stage one of the reclamation process. Then, dried, settled material must be separated and refined to be of use.
Rathnayake and Dawood have devised a new way to remove lithium from petroleum wastewater – a lithium-trapping filter derived from a renewable resource. This proprietary nanomaterial is able to trap molecules of lithium measuring only one to three nanometers, or about a billionth of a meter.

The Greensboro I-Corps program, and then a $50,000 national I-Corps Award, supported Dawood as she conducted field research in the U.S. petroleum industry, including attending a trade show where she was able to access representatives of many companies.
The researchers determined that their filter can be commercially viable when the lithium content in water is 400 milligrams per liter or greater. Dawood learned that amounts of suspended lithium in wastewater range from 400 to 1500 milligrams per liter. The filtering process is faster and more efficient than the evaporative method, able to process up to 55 gallons per minute.
Another advantage of the nano-filter process, Rathnayake says, is that the polymer used to create the filter is naturally abundant, renewable, and therefore relatively inexpensive.
The petroleum industry, always looking for ways to trim costs and boost profits, has shown much interest in the filter, Dawood says, especially in Pennsylvania, North Dakota, and Arkansas. Those states mandate that petroleum production wastewater must be cleansed to remove environmentally harmful material.
Nationally, Dawood said, the petroleum industry annually produces 700 billion barrels of wastewater.
Like the gold dust that attracted prospectors to California in 1849, harvesting minute amounts of lithium can turn into real profits. It doesn’t take long to understand why these researchers are excited about their innovation’s potential.
Dawood, a scientist with an entrepreneur’s passion, hopes to create a company that will license the process and take it to market. She’s also identified firms that she might partner with to achieve the same end.
There’s another fascinating aspect to this nanotech innovation: The polymer that makes up the filter is a conductor. Once packed with lithium particles, the filters essentially become batteries, ready to store electricity.
Rathnayake and Dawood have trademarked a brand name for their nascent product, a name that pays homage to UNCG. It will be called Minerva Lithium.
This article was part of a larger UNCG Research Magazine feature story about campus research and entrepreneurship. Read more at researchmagazine.uncg.edu/spring-2020/its-a-go/.
Story by Tom Lassiter
Photography by Martin W. Kane, University Communications


